Galaxy Risk Suite
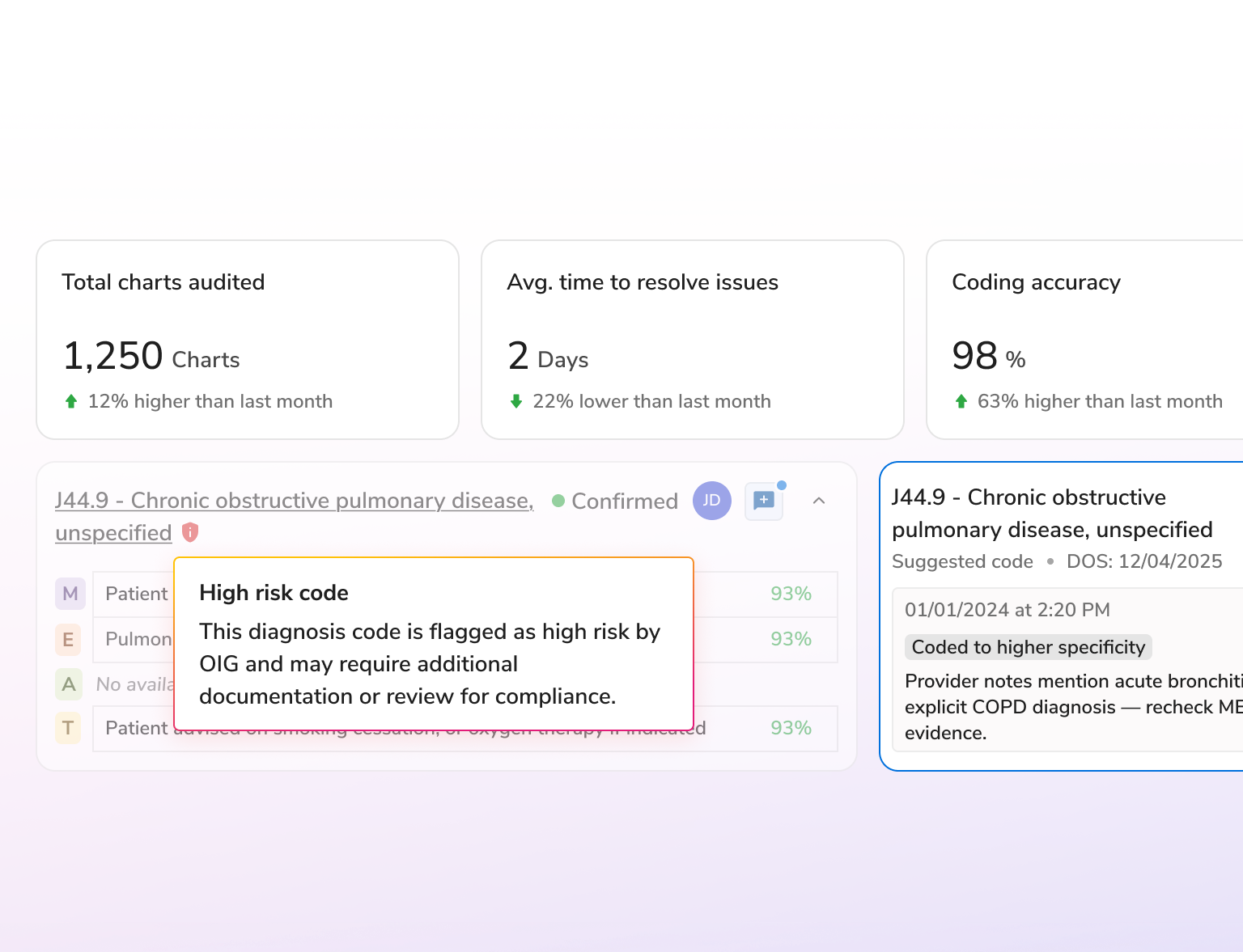
AI-powered risk adjustment for accurate coding, greater efficiency, and audit-ready compliance.
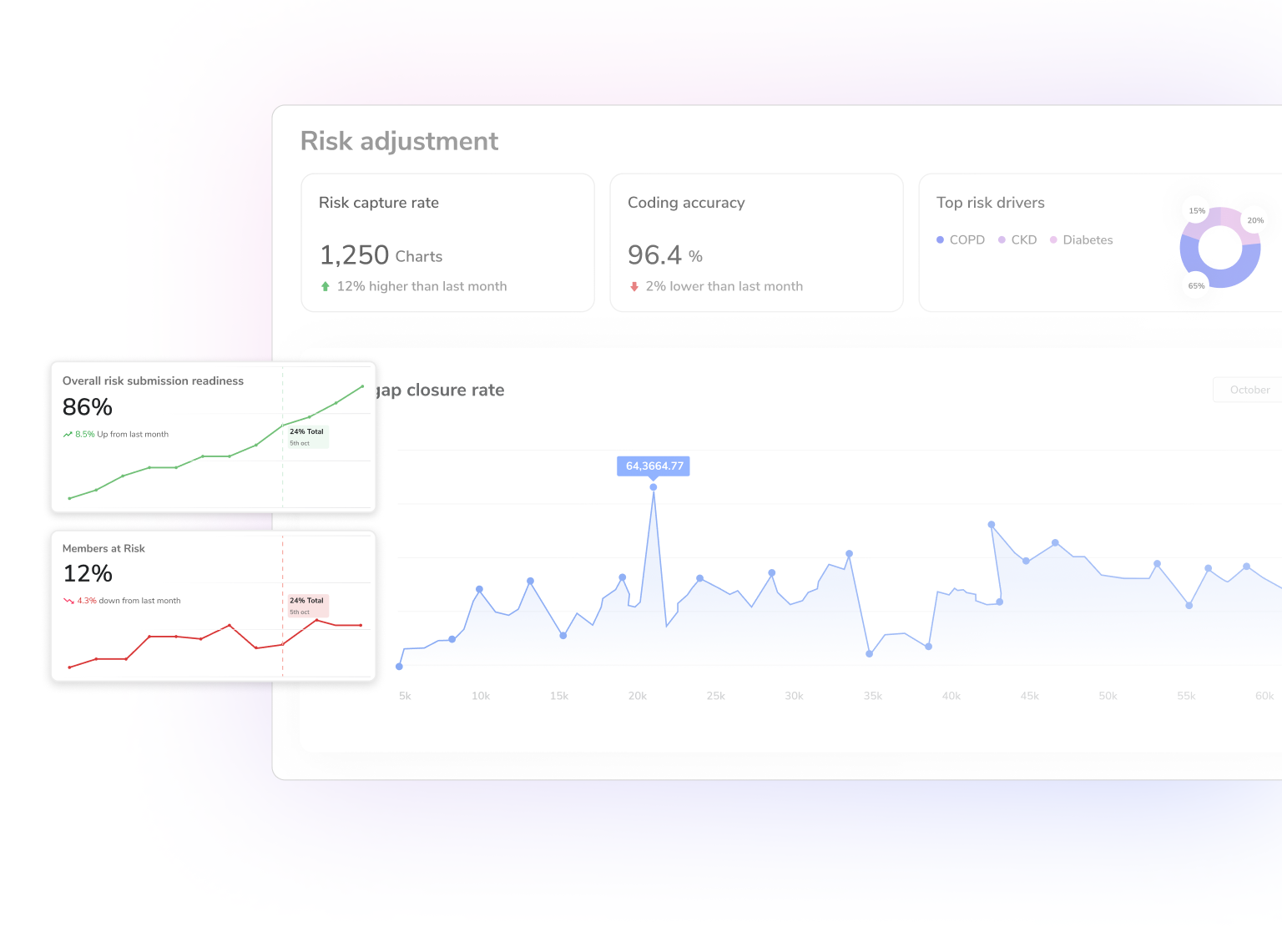
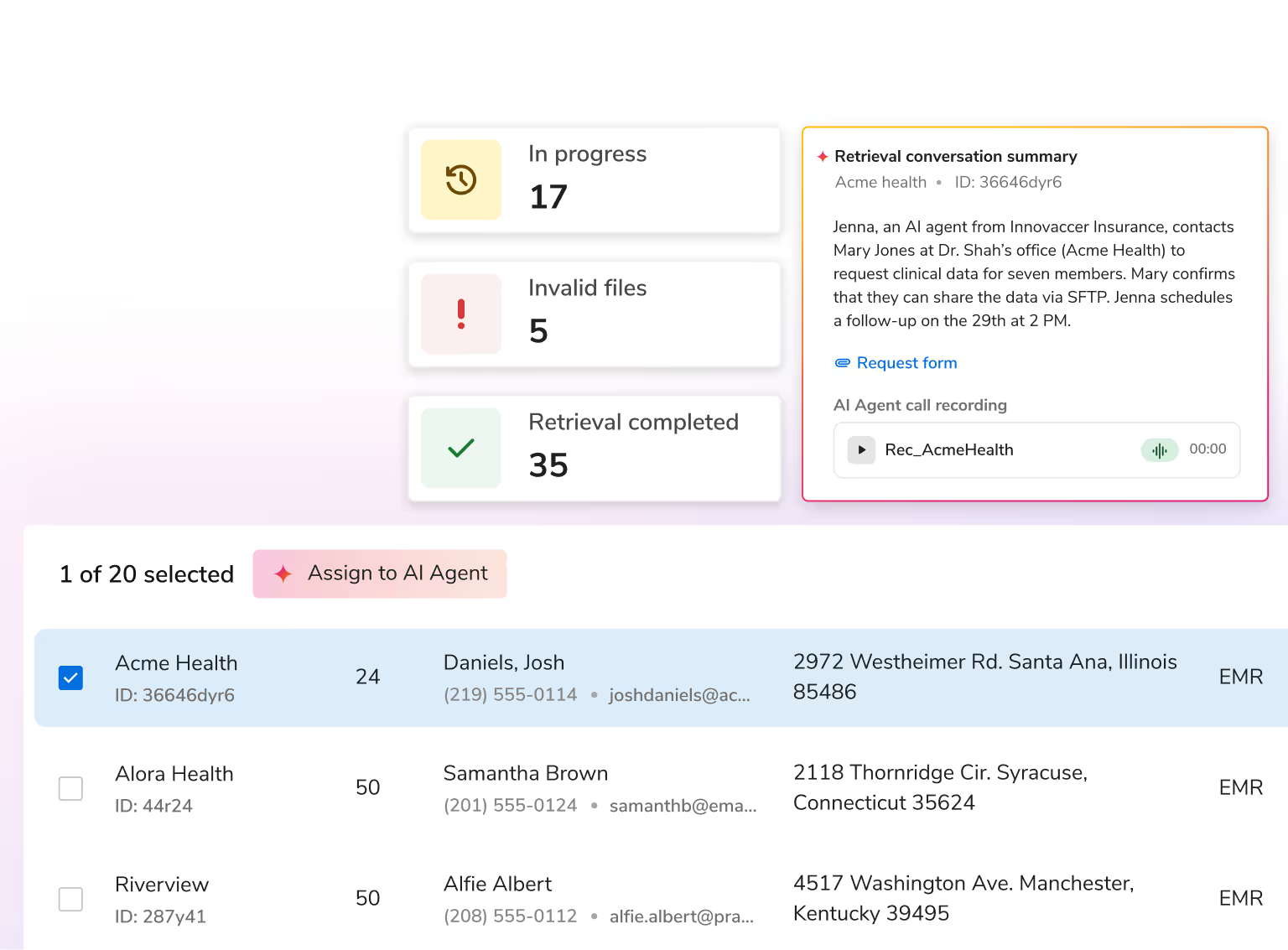
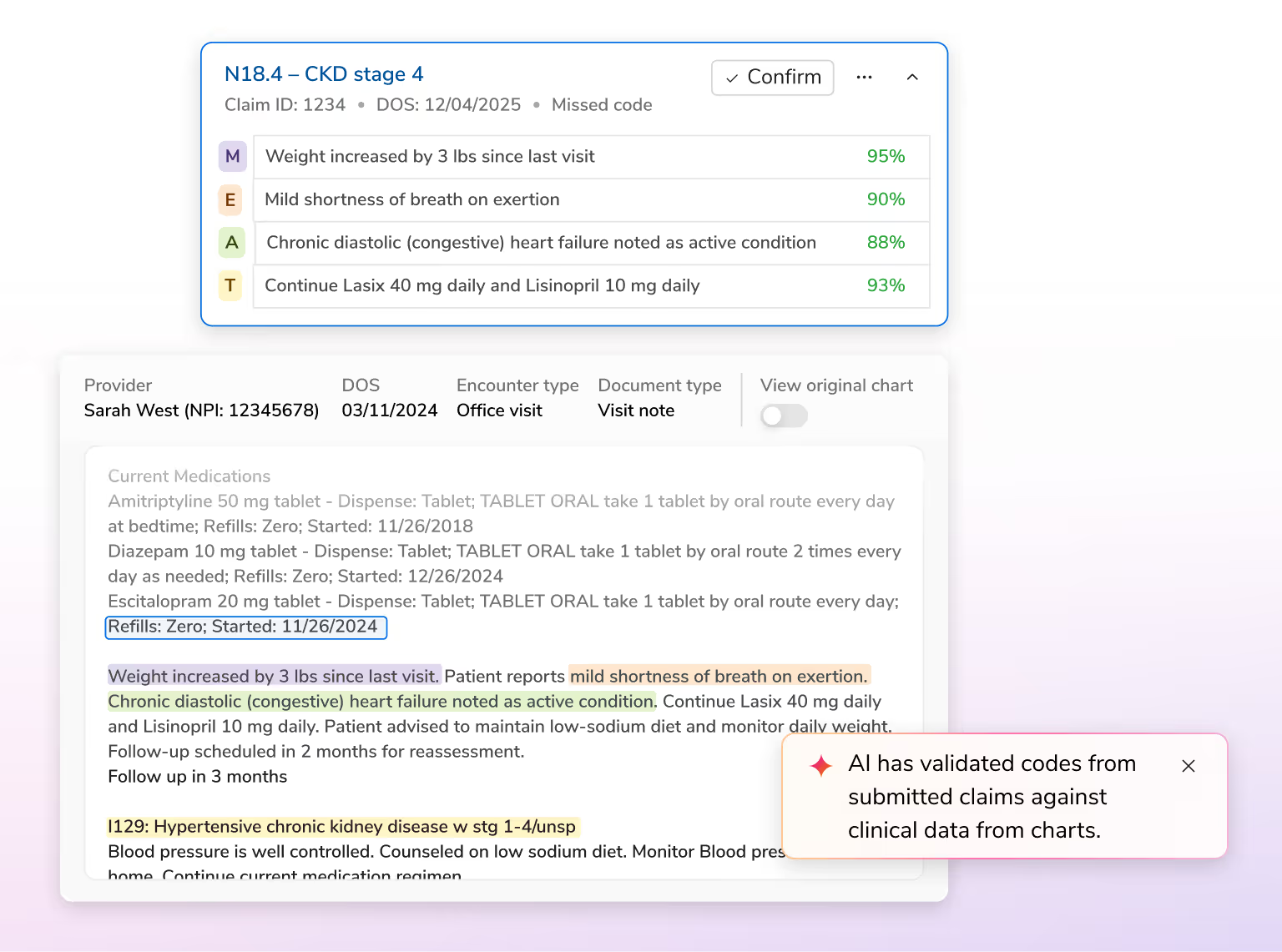
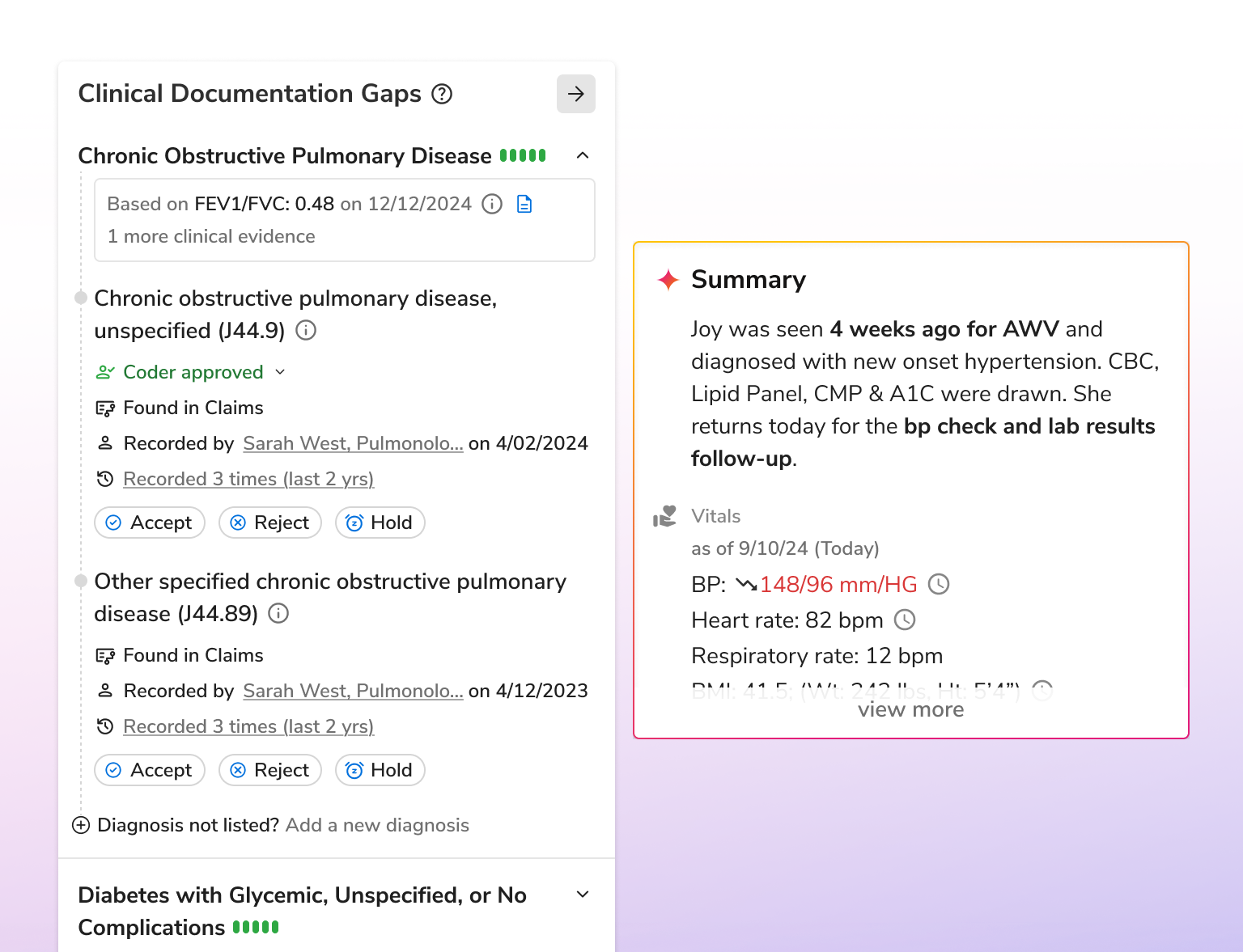
Enhance risk documentation, reduce administrative effort, and save costs with AI that unifies data, automates validation, and drives compliant, efficient workflows across the risk lifecycle.








.svg)